MOSS 100®+ USTICA
MOSS 100®
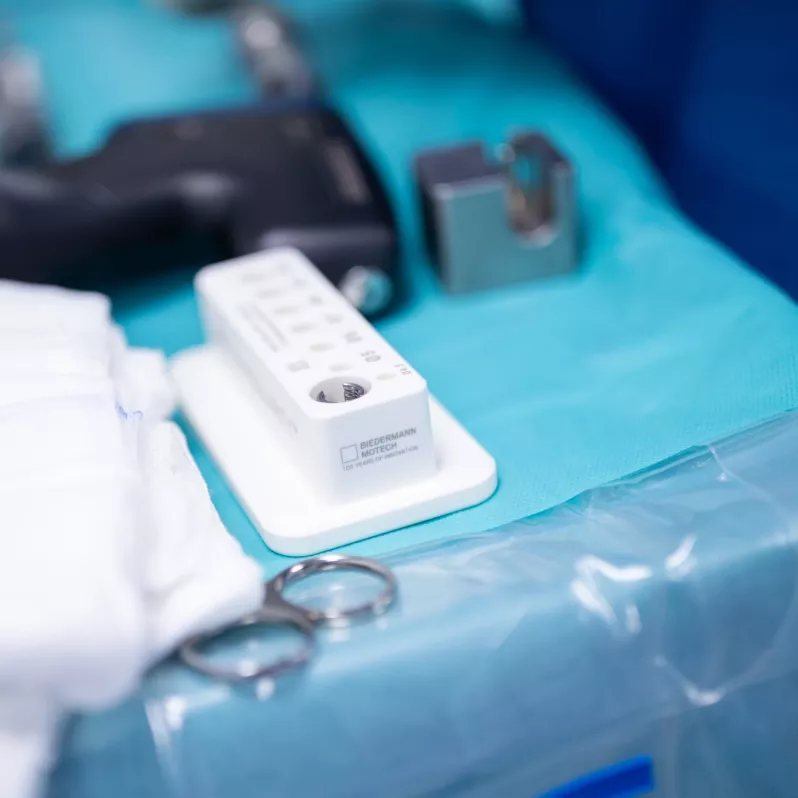
Pedicle screw system for the fixation of thoracolumbar spine segments manufactured by the German company Biedermann Motech.
Who is it for
Adult patients suffering from degenerative disc disease (DDD), spondylolisthesis, pain resulting from trauma, spinal stenosis, tumour, pseudoarthrosis or in the case of a previous failed fusion can become recipients of the implant. The system is not indicated for use in patients on whom it is not possible to carry out imaging procedures intraoperatively (e.g. fluoroscopy).
Why MOSS 100®?
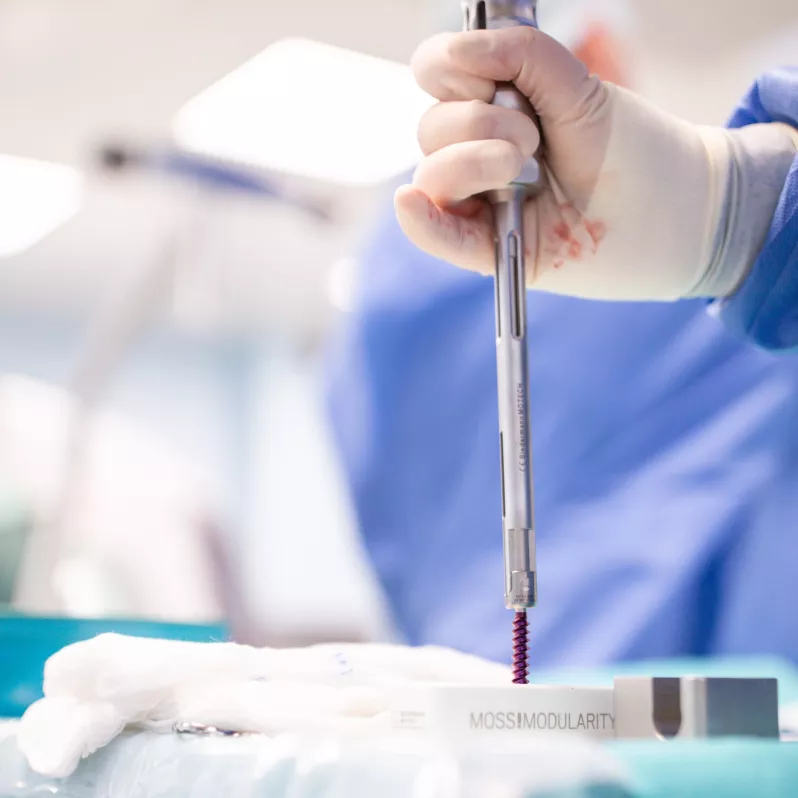
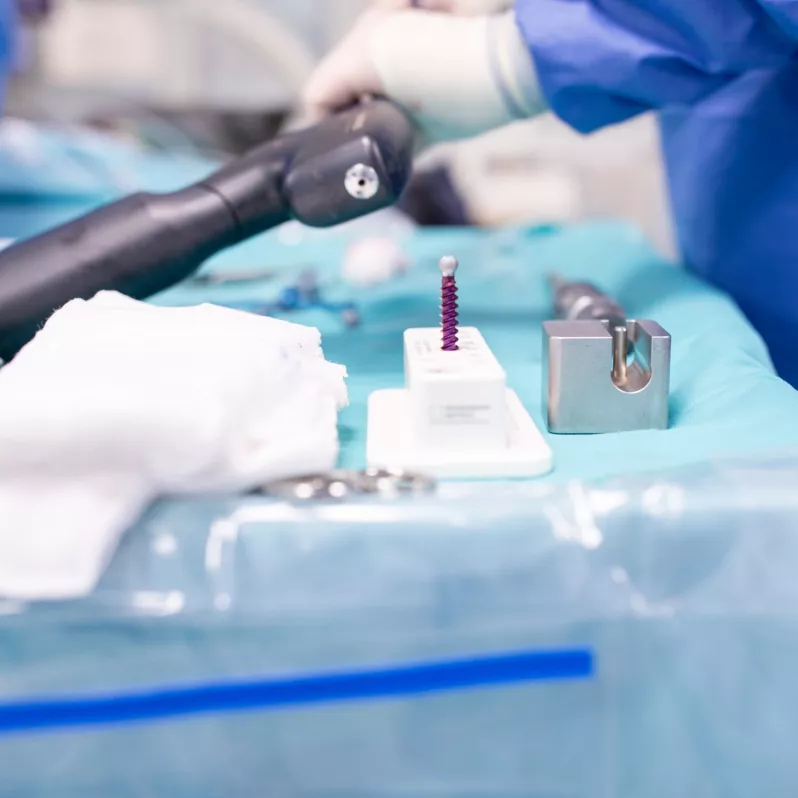
Thanks to its low profile, relatively small polyaxial head diameter and simplified instrumentation, the MOSS 100® ® pedicle screw system facilitates work during surgery when treating mechanical instabilities or spinal deformities. The Friction Fit® feature allows temporary adjustment of the polyaxial screw heads for better rod insertion, further improving the stabilisation capabilities of the implant.
The innovative docking feature of the system contributes to speeding up the entire surgical procedure and provides room for optimal adjustment of the implant to the patient’s anatomical and physiological needs. The entire system is made of a high quality titanium, aluminum and vanadium alloy, characterized by high biocompatibility.
USTICA
USTICA is 3D printed expandable interbody fusion cage from Italian company Tsunami Medical designed to restore and maintain height of the intervertebral bodies and to promote biological fusion.
Who is it for ?
The implant is designed for patients suffering from degenerative disc disease, spinal stenosis, spondylolisthesis, pseudoarthrosis or instability of motion segments in the thoracic, lumbar or lumbosacral regions of the spine, as well as for surgical procedures in case of trauma or tumors. The system is designed to be used in combination with additional means of fixation such as pedicle screws or interlaminar stabilization implants. For patients suffering from osteopenia, osteomalacia or other types of osteoporotic disease, a careful assessment by a surgeon is required before using the implant.
Why USTICA ?
The exceptionality of the Ustica implant lies in its unique internal “net” structure, obtained through an additive manufacturing process. This ensures both excellent primary fixation and sufficient positional stability of the implant with minimal risk of migration. With its elasticity and structural properties, Ustica is close the structure of a healthy bone, which, together with the roughened porous surface contributes significantly to the fast osteo-integration of the implant. Another innovative feature is the multi-directional expansion mechanism, which allows for height expansion as well as changing the desired lordosis angle by expanding in the anterior and posterior direction, making it possible to comfortably adapt Ustica to the anatomical requirements of each patient.
The implant is offered in two transverse-longitudinal footprints with a hight range of 8-13 mm and possible lordosis angles ranging from 2° to 14°.